Introduction:
Alessandro Volta, in full Conte Alessandro Giuseppe Antonio Anastasio Volta, born February 18, 1745, Como, Lombardy [Italy] passed on March 5, 1827, Como), Italian physicist whose creation of the electric battery gave the main wellspring of constant flow. He was the father of an electric cell that became famous in the world to represent an electric cell. The magnificence of his gadget was that nearly anybody could make one silver and copper coins were accessible to many individuals, as were different metals, for example, iron, tin, and zinc. He also made an electric engine from the Volta battery, which was rare for an invention that was impossible. Read the full article to get information from his story.
| Basic Information | Alessandro Volta |
| Nationality | Como |
| Date of Birth | 18th February 1745 |
| Place of Birth | Como, Duchy of Milan |
| Date of Death | 5th March 1827 |
| Place of Death | Como, Lombardy-Venetia Austrian Empire |
| Age | 82 years old |
| Occupation | Inventor, physicist, Chemist |
| Career | 1774-1827 |
| Famous for | The invention of the electric cell |
| Title | Discovery of methane |
| Other works | Actress, film producer |
| Awards | Copley Medal (1794)Legion of HonorOrder of the Iron Crown |
Early Life and Education:

Alessandro Volta was brought into the world in Como, Lombardy, Italy, on February 18, 1745. Alessandro Volta family was essential for the honorability, however not well off. Until the age of four, he gave no indications of talking, and his family dreaded he was not exceptionally savvy or perhaps idiotic. Luckily, their feelings of dread were lost[1].
Alessandro Volta dad died, leaving unpaid obligations when Alessandro was seven. His uncle instructed at home the youthful Alessandro until he was twelve years of age. He at that point began learns at a Jesuit live-in school. The Jesuit school charged no expenses, however, pressurized him to turn into a cleric. His family didn’t need this and pulled out him from the school following four years. Volta at that point learned at the Benzie Seminary until arriving at eighteen years old[2].
Volta’s Family Needs a Lawyer:
Volta’s family needed him to turn into a lawyer. Volta had his thoughts! He was keen on his general surroundings; he needed to be a researcher.
Even though as a youngster he had delayed to communicate in Italian, Volta presently appeared to have an extraordinary ability for dialects. Before he left school, he had learned Latin, French, English, and German. His language gifts helped him in later life when he went with the point of examining his work with researchers in Europe’s communities of science [2].
Testing again and again:
Matured 18, Volta was striking enough to start a trade of letters about power with two driving physicists: Jean-Antoine Nollet in Paris, and Giambatista Beccaria in Turin. Beccaria didn’t care for a portion of Volta’s thoughts and urged him to learn more by doing tests. At the point when he composed his first paper, Volta tended to it and devoted it to Beccaria[2].
Volta’s Career Timeline Before the Battery:
Beginner Scientist, Inventor, Teacher, and Physics Professor:
1765–1769:
Volta had arrived at 20 years old. His well-off companion Giulio Cesare Gattoni had constructed a material science lab in his home. For quite a while he generously permitted Volta to do tests in this research center. He composed his first logical paper, which he routed to Giambatista Beccaria, about electricity produced via friction created by scouring various substances together–for example, triboelectricity.

He distributed an exposition named On the Attractive Force of the Electric Fire, and on the Phenomena Dependent on It, which he shipped off Beccaria. Volta communicate about his thoughts on the reasons for electrical fascination and repugnance and contrasted these and gravity. He set out his position that similar to gravity, electricity produced via friction included activity a good way off. The primary researchers affecting his reasoning were Isaac Newton, Roger Boscovich, Benjamin Franklin, and Giambatista Beccaria himself[2].
1771–1775:
Volta read Joseph Priestley’s 1767 audit of logical examination on power. He discovered that a few revelations he had made as of late had just been made by others. Volta started work administering schools in Como. He said that instructing in Como’s study halls should modernized. He needed the youngsters to invest more energy in learning science and present-day dialects. Volta started instructing trial material science in Como’s public language structure school, where he worked until 1778.
Volta composed a letter to Joseph Priestley. He clarified how he had designed a gadget that created electricity produced via friction: the power could moved to different articles. We consider this gadget the electrophorus. Volta needed to know whether the gadget was another innovation. Clerical revealed to him Johann Wilcke had developed such a gadget in 1762, however, Volta had created it autonomously. Priestley urged Volta to keep up his intriguing examination work[2].
1776–1778:
Aged 31, Volta was the primary individual to segregate methane gas. He found that a methane-air blend could detonate in a shut compartment with an electric sparkle. An electrically begun synthetic response like this would later be the premise of the inside burning motor. Volta recommended that the starting device he used to detonate methane could likewise used to impart an electric sign along a wire from Como to the city of Milan.
Volta created an improved eudiometer than any that had gone previously. A eudiometer tests how much oxygen is available in the air to decide how useful for breathing it is. Volta’s eudiometer was better than others since it used hydrogen as the gas responding with oxygen, giving a spotless, solid response. The Response additionally neatly begun using an electric flash. Eudiometer chipped away at the premise that the volume of hydrogen gas in it diminished in the wake of starting because the hydrogen responded with oxygen gas to make water. The decline in volume was corresponding to the measure of oxygen present noticeable all around.
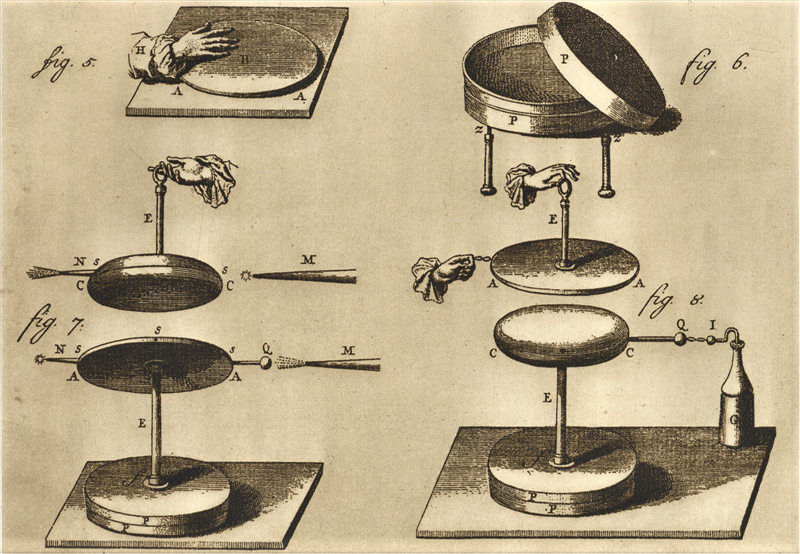
Volta set out on a logical excursion to Switzerland and France. He met different researchers and showed to them his advancements in electrical gear. He likewise went to improve his name known as external Italy. Volta designated to the Chair of Experimental Physics at the University of Pavia, around 55 miles (85 km) from Como, a position he would hold for over 40 years. Volta found that the electrical potential (we presently frequently call this the voltage) in a capacitor is legitimately relative to an electrical charge. [2]
1781 to 1794:
Volta went around a large portion of Europe’s major logical focuses, remembering the French Academy for Paris, exhibiting his electrical gear and developments to prominent individuals, for example, Antoine Lavoisier and Benjamin Franklin. He was turning out to be notable external Italy. He expounded on the condenser he had built (today we would consider it a capacitor) to gather and store electric charge, and how he had used it to contemplate an assortment of electrical wonders. Volta fabricated progressively delicate electroscopes to identify and gauge the affects of electric charge.
Volta probed the conduct of gases. He found an exact incentive for air’s expanding volume with rising temperature. Recognizing that he had gotten one of Europe’s chief electrical researchers, Volta chosen to be a Fellow of the Royal Society of London. At 50, Volta granted the Royal Society’s top prize the Copley Medal for his commitments to logical comprehension of power. [2]
The innovation of the Electric Battery:
A Feud Over Frogs’ Legs Prompted the Battery:
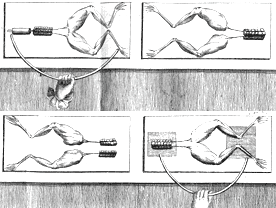
Volta didn’t decide to concoct the battery. His examinations around there performed to show the cases of another researcher weren’t right. That researcher was another Italian, Luigi Galvani.
Galvani was a teacher of life structures. In the last part of the 1780s, he saw that a flash of electricity produced via friction conveyed by a metal surgical tool hitting the sore spots of a dead frog while the legs lay on metal made the legs move. This was a stunning disclosure: creature development depended on power here and there.
In 1817, this propelled Mary Shelley to compose Frankenstein. In this novel, Doctor Frankenstein rejuvenates an animal made of a huge combination of body parts from dead individuals using power from a lightning storm[2].
Method to Produce Current from Liquid:
In 1791, Galvani reported his disclosure of creature power. Volta accepted creatures produced power in their bodies and that a liquid inside their nerves conveyed power to muscles, causing development. He accepted that power from an external source delivered a progression of electrical liquid from the nerves, making the muscles hop.
Volta additionally accepted that creatures, for example, electric eels could develop additional measures of this liquid and use it to convey electric stuns. Galvani presumed creature power was comparable, however not indistinguishable from electricity produced via friction and was a novel property of living things[2].
Enter Volta:
Alessandro Volta Considered Galvani’s Wonder:
In 1792, Alessandro Volta said that the “creature” part of Galvani’s creature power not required. Creatures just reacted to ordinary power. There was no contrast between creature power and power. Volta performed different trials on frogs’ legs. He found the way to getting them to move was contact with two unique metals. Contact with bits of a similar metal sat idle.
At that point, moving ceaselessly from frogs’ legs in 1794, Volta did trials to gauge the electrical impact of bringing various sets of metals into contact. He recorded the metals arranged by what he called their electromotive power[2].
Volta’s List of Conductors, Highest Electromotive Force First:· Zinc
- Lead
- Tin
- Iron
- Copper
- Silver
- Gold
- Graphite
- Manganese Ore

This was the first occasion when anybody had recorded anode possibilities. It was the main electrochemical arrangement. In present-day language, we would state that the farther separated the substances on this rundown are, the more noteworthy the voltage they will deliver when brought into contact or used as the terminals in electric cells and batteries. For instance, a zinc-graphite cell will deliver a more noteworthy voltage than a zinc-lead cell.
By 1797, Volta had showed his “contact hypothesis” of power. He presently realized that the way to delivering what today we call a voltage was two metals associated with something wet, similar to frogs’ legs. The sodden association between the metals didn’t need to be a creature. Interfacing the metals by putting them in a cup of weak corrosive was a viable method of delivering power[2].
Splitting Electrical Channels:
He officially split electrical channels into those of the primary kind: these were metals, graphite, and unadulterated charcoal; and the subsequent kind: these were substances we would now call electrolytes, for example, saltwater or weaken acids. An electric flow would result when a circuit fabricated using two channels of the principal kind joined with one of the subsequent kinds. Interfacing the metals with paper absorbed weak corrosive or saltwater additionally worked. Volta said that in Galvani’s work, the frogs’ legs had served two capacities:
- · They directed power as conductors of the subsequent kind.
- · They went about as an exceptionally touchy electroscope. (An electroscope is a gadget used to identify power.)
Volta found that by associating up an ever-increasing number of sets of metals associated with a wet card, he could deliver ever higher voltages, prompting huge electrical flows. Thus, the electrical battery perceived. Volta used substituting zinc and silver circles connected via card or fabric absorbed saltwater[2].
Volta’s Joining of Royal’s Family:
In 1800, Alessandro Voltadepicted his outcomes in a letter to Joseph Banks, at the Royal Society in London. Banks showed the letter to different researchers and masterminded Volta’s depiction of his revelation to be perused out at a gathering of the Society and afterward distributed[2].
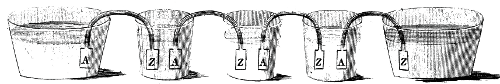
Wave of New Scientific Discoveries:
The battery Volta imagined gave scientists an exceptionally incredible new technique to contemplate substances. The magnificence of his gadget was that nearly anybody could make one silver and copper coins were accessible to many individuals, as were different metals, for example, iron, tin, and zinc.
Inside long stretches of Volta’s development of the battery, William Nicholson and Anthony Carlisle manufactured and used a battery to decay water into hydrogen and oxygen. Inside only six years, Humphry Davy had manufactured a ground-breaking battery. With it, he separated new synthetic components and concluded that substance securities were electrical[2].
Davy’s Revelations of the New Components:
Volta’s creation of the battery completely made Davy’s revelations of the new component’s barium, calcium, lithium, magnesium, potassium, sodium, and strontium, conceivable. By 1820, politeness of Volta’s batteries, Hans Christian Oersted was researching the connection between power and attraction[2].
Formation of Electrical Engines:
By 1821, Michael Faraday had created an electric engine. Volta’s battery created a consistent wellspring of electric flow unexpectedly. All electrical gadgets rely upon electric flow. Without Volta’s innovation, there could be no advanced innovation. Volta’s battery was a vital innovation in the advancement of our innovation-based progress.
In 1819, at 74 years old, Volta concluded the time hung up his capacitors, his voltaic heaps, his electrophorus, and his authoritative work at the college. He resigned to a nation house near his old neighborhood of Como, where he could invest more energy with his significant other, Maria Teresa. They had three children, Zunino, Flaminio, and Luigi[2].
Volta’s Death and Later:
Alessandro Volta lived in Como until his demise, matured 82, on 5th March 1827. In 1881, researchers concluded that the unit of electric potential would known as the volt to perceive Volta’s extraordinary commitments to electrical science[2].
References:
1. 24th November 2020; Available from: britanicca
2. 24th November 2020; Available from: famousscientists