Introduction:
Henry Mosley was an English Physicist, who pays to the discipline of astronomy was the explanation from the rules of the preceding experiential and the organic idea of the submicroscopic amount. This restricted beginning his growth of Moseley’s law in X-ray Scales. J. Robert Oppenheimer made an atomic bomb that was used for the high destruction and other items like Nuclear Weapons development, Tolman Oppenheimer Volkoff limit, Oppenheimer–Phillips process, and Born Oppenheimer, approximation.

| Basic Information | Henry Moseley |
| Nationality | British |
| Date of Birth | 23rd November 1887 |
| Place of Birth | Weymouth, Dorset, England |
| Date of Death | 10th August 1915, |
| Place of Death | Gallipoli, Ottoman Turkey |
| Cause of Death | Killed in an action |
| Age | 27 years old. |
| School | Summer Fields School |
| High School / College | Trinity College |
| University | The University of Oxford, The University of Manchester |
| Occupation | Scientist, Inventor, Physics, and Chemistry |
| Career | 1910 – 1915 |
| Famous for | Atomic number, Moseley’s law |
| Title | The inventor of atomic number |
| Other works | X-ray Spectrometer |
| Awards | Matteucci Medal (1919) |
Early Life and Education:
Henry Gwyn Jeffreys Mosely, born in the town of Weymouth, England, in the UK on 23rd November 1887. His folks were both from accomplished families. The father of Mosely, his name was Henry, was a teacher of life structures and physiology. His mom, Amabel, was the little girl of a counselor who had changed professions to turn into a mollusk scholar [1].

Henry Moseley got an education in tuition-based schools. His first school was Summer Fields School–a grade school. There he won a grant for Eton College, which is presumably Britain’s most renowned secondary school. In the wake of showing up at Eton, he chose the school’s material science exercises that were excessively simple. So he dealt with the subject autonomously. Matured 18, he won Eton’s material science and science prizes [1].
Effectively a high achiever, he got admission in 1906 to the University of Oxford’s Trinity College, where he examined material science. There, he frustrated himself. He was experiencing serious hay fever when he sat on his end-of-the-year tests. He got a below-average distinctions degree in material science, not the ‘primary’ he had sought after and expected [1].
Ernest Rutherford’s Laboratory:
In 1910 Moseley moved to the University of Manchester to join Ernest Rutherford’s examination gathering. Rutherford had become world acclaimed two years sooner when he was granted the Nobel Prize in Chemistry for his disclosures in radioactivity. Even though Moseley’s degree was ‘one moment,’ Rutherford took him on after getting with teachers at Oxford that he was a promising physicist. Their characters were contrary energies. Rutherford was garrulous and noisy, while Moseley fairly held, using no greater number of words than he discovered vital [2].
(Rutherford appears to have been a noisy character, so boisterous that partners, for example, Geoffrey Fellows poked fun at it.) [2]

At Manchester, Moseley instructed material science and completed examination work. He before long discovered that he didn’t appreciate showing a side of his work. After Moseley had been at Manchester for a year, something adequately dazzles Rutherford with his work that he offers him an examination association. Moseley acknowledged this cheerfully–it permitted him to focus all his consideration on exploration and drop his educating work [2].
Henry Moseley’s Experiments:
The Atomic Battery:
Working in Rutherford’s gathering, it was inescapable that Moseley would work with radioactive compound components. After getting comfortable with the field, he went past analyzing others had thought of and concocted his specific wind. In 1912 he endeavored to use high sure voltages to pull beta particles (high-energy electrons) back into their radioactive source. (This seems like a pleasant thing at any rate. Yet Moseley wanted to use the outcomes to reveal insight into one expectation of Albert Einstein’s extraordinary hypothesis of relativity: that mass increments with speed.) [2]
He attempted to pull the beta particles back by protecting their radioactive source (radium) so it would be progressively certain as the beta particles diverted negative charge [2].
Henry Moseley was a remarkably smart exploratory physicist. In 1913 he used self-manufactured hardware to show that the number of protons it has remarkably dictated each component’s personality. His revelation uncovered the genuine premise of the intermittent table and empowered Moseley to foresee unhesitatingly the presence of four new substance components, which were all found [2].
Beta Emission:
If the radium could arrive at an electric capability of 1,000,000 volts, at that point something would pull once again even the most vivacious beta particles into the source as they discharged. Lamentably, the serious extent of flawlessness required in protecting the radium couldn’t be accomplished, so 1,000,000 volts couldn’t be reached [2].
Notwithstanding, by producing voltages (around 150,000 volts) on a radioactive source, Moseley made the world’s first nuclear battery–a beta cell. He considered it a radium battery. Today, nuclear batteries are used where long battery life is vital, for example, in heart pacemakers and rockets [2].
Explanation of the Periodic Table:
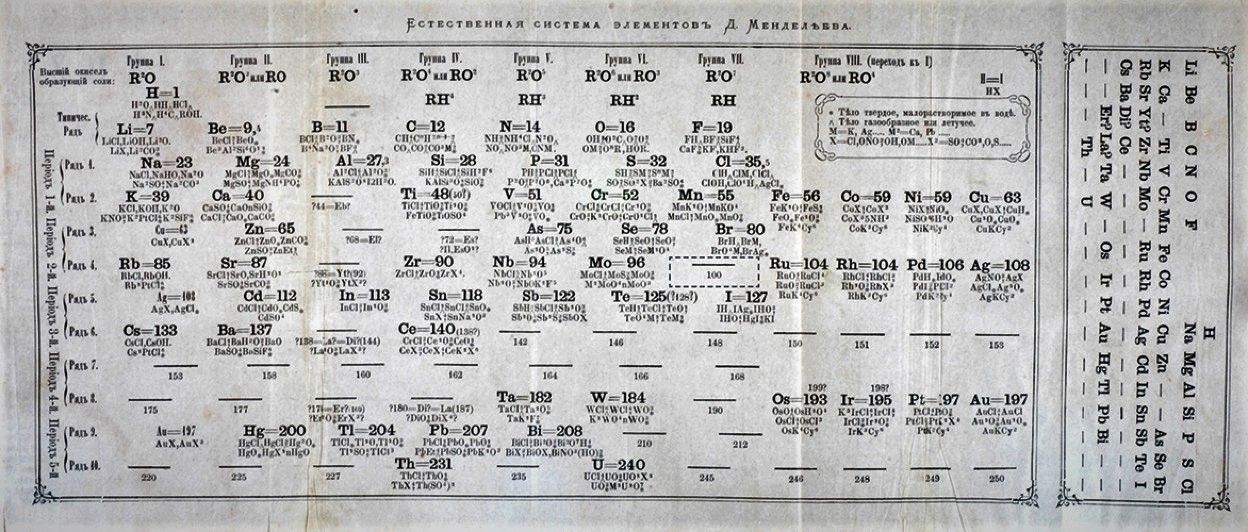
In the year 1913, Moseley celebrated his 26th birthday. Dmitri Mendeleev’s periodic table became older, and it remained for about 44 years. After that, the additional elements are extracted and added to the table. Subsequently, Mendeleev’s table was been used and the periodic table had rendered to the atomic weights and the Chemical belongings [3].
There was an essential defect in the table: the position expected by a component’s nuclear weight rarely coordinated the position expected by its synthetic properties. In these cases, components were in the occasional table as per their properties, instead of their nuclear weight. Was it conceivable that components could have more principal property than nuclear weight [2].
Antonius van cave Broek’s Hypothesis:
In 1911, Antonius van cave Broek had distributed his theory to that nuclear number–which right now was just the situation of a component. In the occasional table–may be equivalent to the measure of charge in the particle’s core. There was no test proof to show this speculation [2].
Shooting Electrons at the Elements:
Moseley had gained from William and Lawrence Bragg that when high-energy electrons hit solids, for example, metals, the solids produce X-beams. This fascinated Moseley, who contemplated whether he could consider these X-beams to become familiar with what goes on inside iotas; he had van nook Broek’s speculation as a top priority explicitly [2].
He moved back to Oxford in 1913. Rutherford had offered him another cooperation at Manchester on a better footing. However, Moseley chose the best way for his vocation is to get involved with few distinct research centers. There was no association open at Oxford, yet Moseley establishes one was coming up. He increased his research center, yet needed to self-reserve his work [2].
In an exceptionally limited quantity of time, he set up a test device to shoot high-energy electrons at various synthetic components and measure the frequency and frequencies of the subsequent X-beams. He found that every component discharges X-beams at an extraordinary recurrence. He additionally discovered he could get a straight-line chart by plotting the square-foundation of X-Beam recurrence against components’ nuclear numbers [2].
His information appeared well and good if the positive charge in the nuclear core expanded by precisely one unit as you look starting with one component then onto the next in the intermittent table. He found that a component’s nuclear number is indistinguishable from the number of protons it has [2].
Synthetic Elements = Proton Numbers:
This was immensely significant. It implied Moseley had found that the fundamental distinction between components is the number of protons they have. He understood the number of protons characterizes a component. If a component has one proton, it must be hydrogen; two protons must be helium, three protons must be lithium, and so forth, and so on even though this may appear glaringly clear to us today, it was a gigantic disclosure in 1913 [2].
At the point when Moseley organized the components in the intermittent table by their number of protons instead of their nuclear loads, the blemishes in the occasional table that had been making researchers awkward for quite a long time vanished [2].
Four New Chemical Elements:
Besides, much the same as Mendeleev had completed 44 years sooner, Moseley saw holes in his new occasional table. He expected four new components, with 43, 61, 72, and 75 protons. Different researchers found later these components; we presently call them technetium, promethium, hafnium, and rhenium [2].
New Method of Identifying Elements:
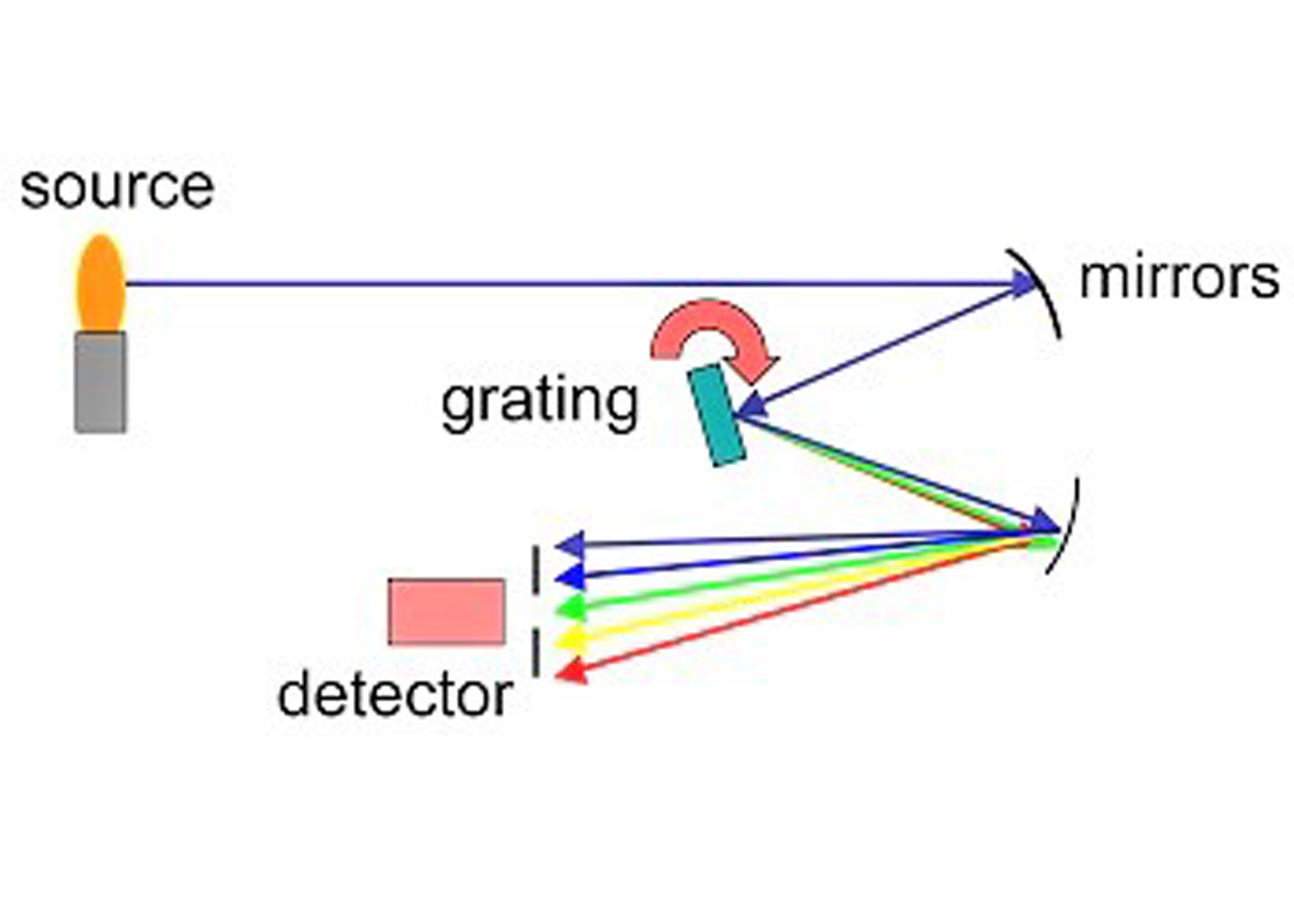
As though his clarification of the intermittent table was insufficient. Moseley had additionally found another non-damaging strategy to discover which components are available in any example. You surround the example with high-energy electrons and take a gander at the frequencies of the subsequent X-beams. These X-beams are tantamount to a unique mark for any components present in the example [2].
At that point, this was an especially welcome method for uncommon earth physicists. Who had discovered their work getting practically horrible? The uncommon earth metals act so comparably that to investigate an example containing these components could take long periods of work. Moseley could now do it in minutes! [2]
X-Beam spectroscopy is currently in use in research centers everywhere in the world. It is likewise used to examine different universes, for example, Mars [2].
Death and the last days:

In 1914, Rutherford and Bragg prescribed to the University of Oxford that Moseley ought to select a seat of material science that getting empty there. Moseley had different thoughts. At the point when World War 1 started in 1914, he enrolled as a volunteer in the British Army’s Royal Engineers. His family begged him to proceed with his logical examination, and the military was hesitant to acknowledge him. Moseley needed to contend energetically to get into the military [2].
They killed second Lieutenant Henry Moseley fighting at 27 years old in Gallipoli, Turkey on 10th August 1915. His grave is on Turkey’s Gallipoli Peninsula [2].
Awards:
After his death, in the year 1916, they did not award him the Nobel prize for his fruitful works in physics and chemistry. There is a solid technical agreement that Henry Moseley, had been alive, would take conventional one of these awards [2].
Conclusion:
Henry Moseley discovered the atomic number which was a brilliant invention till today. Formation of the atomic number tells us the atomic mass, neutrons, protons, and the nucleus due to which we can say that Na (sodium), Li (lithium), or any other which is now used in the periodic table. The periodic table is arranged in such a way that each number placed in ascending order. In nowadays, we use these atoms for the production of different things like making metals of different atoms and their atomic masses. J. Robert Oppenheimer, which was very explosive, invented the atom. The primary purpose of the atom bomb is to protect the country from outer danger and not to use it for killing those humans who are not involved in criminal activity and are not involved in the support of them.
References:
- en.wikipedia. 28th October 2020; Available from: https://en.wikipedia.org/wiki/Henry_Moseley#Scientific_work.
- famousscientists. 28th October 2020; Available from: https://www.famousscientists.org/henry-moseley/
4 Replies to “The inventor of the Atomic Number and the Periodic Table, Henry Mosley”